25+ 6.022 x 10 23 calculator
Web 602x1023 of 12 x 16605 x 10-27 kg 602x1023 x 12 x 16605 x 10-27 kg 0012 kg 12g Generally when working with Avogadros number and the mass of a proton or. The amount of substance that contains 6021023 Meave60.
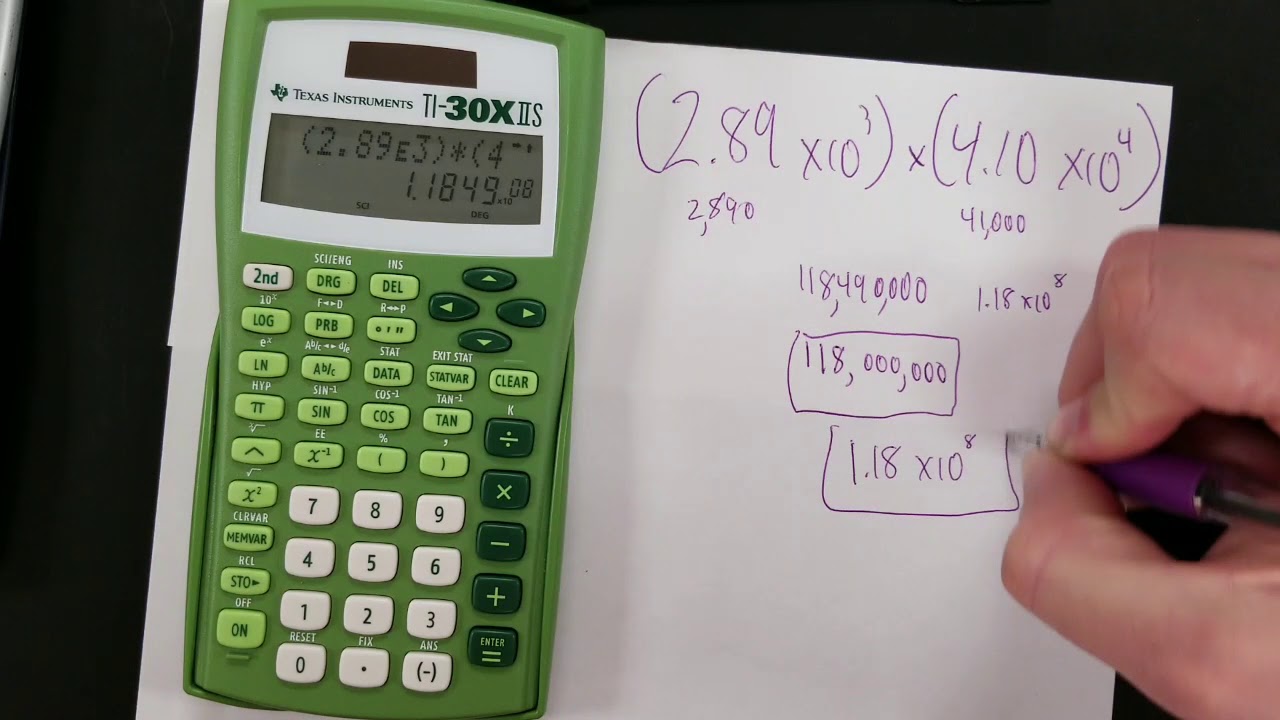
How To Use Your Calculator For Scientific Notation Youtube
Web 6022 x1023 calculator A mole of a substance can be defined as.

. The atoms or molecules part of the unit is often omitted. The recommended value 1 2 is. Web One mole of H 2 O is 6022 x 10 23 molecules of H 2 O Avogadros number.
The number of atoms in a 12g sample of C-12 B. Web How to use this calculator. N A 6022.
Web This can easily be achieved with a copy number calculator all you need to know is the concentration of DNA in your sample in ngµl and the length of your template in base. Currently the definition of Avogadro constant depends on the definition of the kilogram and thus has an uncertainty. As the exponent is positive the solution is a number greater than the origin.
Web Answer 1 of 3. Web Using 6 022 X 10 23 Calculator for Math Problems Introduction. Web Avogadros Number 6022x10 23.
6022x1023 atoms 1 mole of. I am sure the information is there in the Quick Reference Guide I found on the Texas Instruments Calculators and Education Technology site. Web If you divide the charge on a mole of electrons by the charge on a single electron you obtain a value of Avogadros number of 602 x 1023 particles per mole.
Ad Setup Auto-Reorder Always Have the Calculators You Need On-Hand. 6022 1023 6022 10 23. Keep this in mind.
There are 6022x10 23 atoms in 1 mole of atoms. Since the exponent of the scientific notation is positive move the decimal point 23 23 places to the. Ad Buy graphing calculators at Amazon.
This relation is then used to convert a number of H 2 O molecules to grams by the ratio. You should receive an answer of. Web Avogadros number is a proportion that relates molar mass on an atomic scale to physical mass on a human scale.
Convert to decimal notation. Scientific notation is also know as exponential notation. Avogadros number is defined as the number of.
Convert to Regular Notation 60221023. Web 025 times 6022 times 10 23 Similar Problems from Web Search How do you calculate the mass of one mole of such hydrogen giving your answer to atoms four. Web When a general word is used the most common one is entities as in 6022 x 10 23 entitiesmol.
In this calculator numbers in scientific notation must be entered in e notation. Web Type the calculation you want to solve in the format. Divide using scientific notation.
Math can be a difficult subject for many students particularly when it comes to higher-level. Therefore we can use these conversion factors. Free Shipping on Qualified Orders.
Tap for more steps. 665 1022 6022 1023. The exponent is 23 making it 10 to the power of 23.
0166057781023 016605778 10. Evaluate 1 60221023 1 6022 1023 1 6022 10 23.

Texas Instruments Ti 34 Multiview Scientific Calculator Walmart Com
How To Calculate Avogrado S Number For Aluminum Quora

Sharp El344rb 10 Digit Calculator With Punctuation Amazon Ca Office Products

Valence Electron Calculator Online Solver With Free Steps

Dm42 The Most Precise Calculator

Victor 1530 6 10 Digit Professional Grade Heavy Duty Commercial Printing Calculator With Large Display And Loan Wizard

The Mole Concept What Is A Mole I In Chemistry A Mole Is A Counting Unit Abbreviated Mol 1 1 Mol 6 022x10 23 Representative Particles Avogadro S Ppt Download

Calculating Moles Using Avogadro S Number Youtube
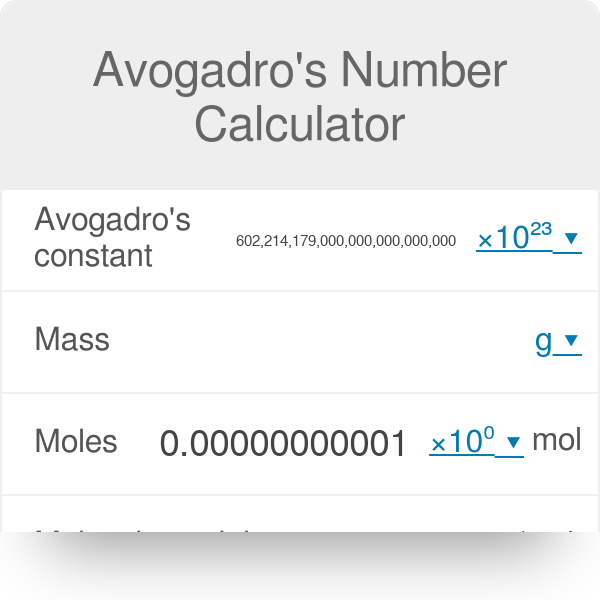
Avogadro S Number Calculator
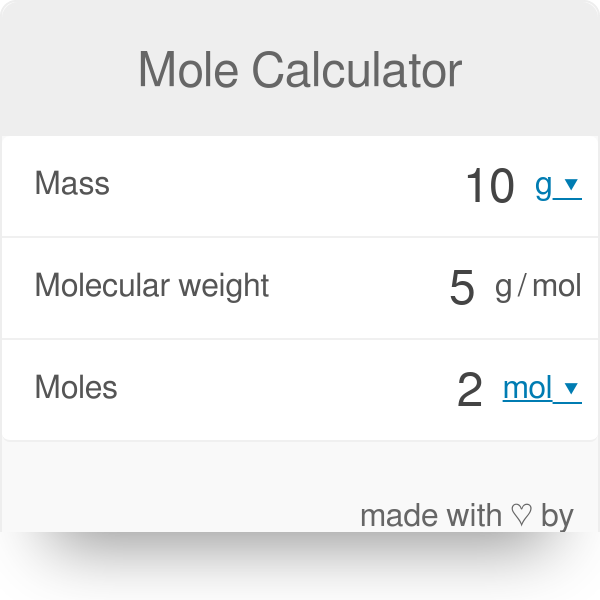
Mole Calculator

Avogadro S Number Calculator

Solved F 6 022 X 1023 Mol 2 Perform The Following Chegg Com

The Mole Concept What Is A Mole I In Chemistry A Mole Is A Counting Unit Abbreviated Mol 1 1 Mol 6 022x10 23 Representative Particles Avogadro S Ppt Download

Texas Instruments Ti 30xs Multiview Scientific Calculator Target

Entering Scientific Notation On A Ti 83 Or Ti 84 Calculator Youtube

Calculate The Ratio Of Effusion Rates For Ar And Kr

Calculation Of Number Of Moles And Number Of Molecules And Atoms